The National Agency for Food and Drug Administration and Control (NAFDAC) has raised alarm over falsified versions of two critical medicines currently circulating in Nigeria.
The agency warned that the counterfeit drugs pose severe risks to public health.
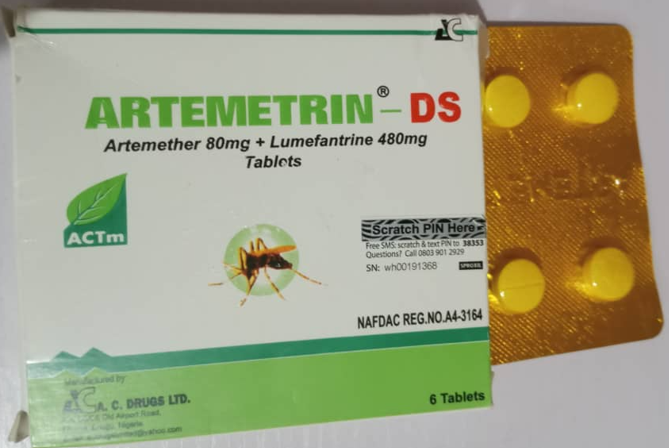
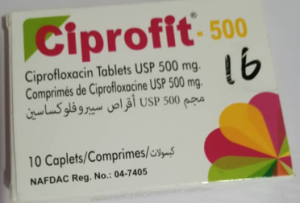
In a public notice numbered 030/2025, NAFDAC identified fake batches of the anti-malarial Artemetrin DS and the antibiotic Ciprofit 500.
The agency said the products were traced to licensed vendors and wholesalers, making detection even more difficult for unsuspecting consumers.
Laboratory analysis revealed that the fake drugs contain dangerously low amounts of active ingredients.
According to the report, Artemetrin DS, labeled as manufactured by A.C. Drugs Ltd in Enugu, contained only 59.2% artemether and 71.2% lumefantrine. This falls below the standard potency range of 90–110%.
Similarly, Ciprofit 500, falsely marked as a product of Impact Pharmaceutical Ltd, was discovered to contain just 5.7% ciprofloxacin.
Neither of these drugs is officially registered in NAFDAC’s database, and the numbers printed on their packaging were fabricated.
“The dangers of substandard anti-malarials in a malaria-endemic country like Nigeria cannot be overstated,” a NAFDAC spokesperson warned.
“These falsified products compromise treatment effectiveness and can result in preventable deaths.”
The regulator advised Nigerians to immediately stop using these medicines and return any stock to the nearest NAFDAC office.
Patients who may have taken the drugs and experienced adverse reactions or treatment failure were urged to seek urgent medical care.
The World Health Organisation (WHO) estimates that about 10% of medicines worldwide are substandard or falsified.
Experts believe the percentage could be even higher in Nigeria, where malaria kills over 200,000 people every year, and bacterial infections continue to threaten public health.
This latest alert follows NAFDAC’s seizure of fake malaria drugs worth ₦1.2 billion in Lagos earlier this month.
The agency has also issued warnings in 2025 about counterfeit Postinor-2 contraceptives, Oxytocin injections, and falsified milk products.
Healthcare professionals, pharmacists, and distributors were urged to verify product authenticity using NAFDAC’s online database.
The public was also encouraged to purchase medicines only from approved outlets and report suspicious cases through the agency’s hotline or email.
NAFDAC’s Director-General, Prof. Mojisola Adeyeye, reassured Nigerians of the agency’s commitment to eliminating counterfeit medicines.
“With government support, we will intensify surveillance and enforcement to reduce fake medicines in circulation to less than five percent by the end of 2025,” she said.